International Journal of Cosmetic Science
M. C. Iglesias de la Cruz*, F. Sanz-Rodrıguez*, A. Zamarron*, E. Reyes , E. Carrasco*, S. Gonzalez,§ and A. Juarranz* *Departamento de Biolog ́ıa, Facultad de Ciencias, Universidad Auto ́noma de Madrid, C/ Darwin, 2. 28049 Madrid, Spain, Industrial Farmace ́utica Cantabria, C/ Arequipa, 1, 28043 Madrid, Spain, Dermatology Service, Memorial Sloan-Kettering Cancer Center, 1275 York Avenue, New York, NY 10065, U.S.A. and §Dermatology Group, O’Donnell 41, 28009 Madrid, Spain
Synopsis
Regenerative properties of skin decrease with age, and thus, the search for substances that minimize cutaneous ageing has increased in the last few years. The secretion of the mollusc Cryptomphalus Aspersa (SCA) is a natural product that bears regenerative properties when applied topically. The purpose of this work is to study the in vitro effects of SCA on cell proliferation and migration, as well as on cell–cell (E-cadherin and b-catenin) and cell–substrate (vinculin and b1-integrin) adhesion proteins expression, using a human keratinocyte cell line (HaCaT cells) and primary dermal fibroblasts (HF). We tested the effects of SCA on cell proliferation using a colorimetric assay. In addition, SCA induced changes on cell migration were studied by woundhealing assays. Besides, Western blot and immunofluorescence microscopy were carried out to test the expression of different cell adhesion proteins. We found that SCA promotes proliferation and migration of HaCaT cells in a time and dose dependent manner. Moreover, treatment with SCA increases the migratory behaviour and the expression of adhesion molecules in both HaCaT and HF. Finally, SCA also improves cell survival and promotes phosphorylation of FAK and nuclear localization of b-catenin. These results shed light on the molecular mechanisms underlying the regenerative properties of SCA, based on its promoting effect on skin cell migration, proliferation and survival. Moreover, these results support future clinical uses of SCA in the regeneration of wounded tissues.
Introduction
Skin degeneration is a complex process induced by ageing and environmental damage, for example UV light. When considering the skin, UV light is the most important damaging factor, leading to skin photoageing and cancer. The main mechanism of UV induced photoageing is a marked increase in the production of reactive oxygen species (ROS), which causes photo-oxidative dam- age and decreases cell migration and proliferation [1, 2]. Photoaging is characterized by the appearance of wrinkles, loss of structural integrity and impaired wound healing [3]. These effects are closely related with alterations in the remodelling process of the extracellular matrix (ECM), a task carried out by dermal fibro-blasts [4, 5].
When the skin is injured by any external aggression, the machin- ery to repair the damage is rapidly activated, including fibroblast proliferation and migration to the wound site to secrete and assemble new ECM components (i.e. collagen, elastin and fibronectin) that renew elasticity and consistency of the skin. In healthy skin, the basal keratinocyte layer attaches to a carpet of specialized matrix, the basal lamina. Therefore, physiological regenerative events include proliferation of keratinocytes and the establishment of new cell–cell and cell–substrate (basal lamina) adhesions. One of the most important molecules involved in intercellular attachment is E- cadherin (ECD), a transmembrane protein that mediates epithelial cell–cell adhesion through other intracellular anchor proteins such as b-catenin in adhesion junctions [6]. Keratinocytes also establish hemidesmosomes, which anchors to the laminin in the basal lamina through specific integrins belonging to the b1 subfamily [7]. In these cells, hemidesmosomes connect the ECM to the intermediate filament cytoskeleton (cytokeratins) inside the cell. These interac- tions are crucial not only for the integrity of the skin but also for signal transduction leading to cell migration and proliferation, for example phosphorylation of the Tyr kinase focal adhesion kinase (FAK) [7, 8]. Finally, survival signals are also needed to maintain the integrity of the new repaired tissue. In this sense, elevated levels of nuclear b-catenin also have been associated with improved cell survival [9]. Among the plethora of signals involved in cellular proliferation, b-catenin translocation to the nucleus plays a pivotal role. In the nucleus, b-catenin is incorporated to the transcriptional machinery, stimulating the expression of proliferation inducing gene products [10].
Research of natural substances that can improve and stimulate skin regeneration has increased in the last few years. In this regard, the secretion of some snails has proven beneficial in the regeneration of burnt skin [11] and in the management of open wounds [12]. In particular, a secretion from the mollusc Cryptomphalus aspersa (SCA) has been proven to have antioxidant activity [13] and to induce skin regeneration after wound-healing impairment from acute radiodermatitis [14]. Moreover, a recent study demonstrated that SCA stimulates fibroblast proliferation, rearrangement of the actin cytoskeleton and stimulates ECM assembly [15]. However, the molecular mechanisms underlying the regenerative properties of SCA are not completely understood. In the present study, we have investigated the regenerative properties of SCA using different in vitro approaches. We found that SCA increased fibroblast and keratinocyte migration and increased the expression of cell–cell and cell–substrate adhesion molecules in both cell types. Finally, we demonstrated a role for SCA in cell survival signalling pathways, because it induces the expression of mol- ecules related with this process, such as active b-catenin, FAK and phosphorylated FAK. Taken together, our results support the use of SCA as a putative cutaneous regenerative product.
Material and methods
SCA preparation
SCA was prepared according to US patent US 5538740. Briefly, the gastropod was physically stimulated by centrifugation to increase the secretion naturally produced by the mucinous, albuminous and salivary glands. Then, the secreted fluids were separated and collected from the live gastropod, clarified by centrifugation and further clarified by filtration through 0.22-lm filters. Further dilutions were performed in aqueous solution (pH 7.4). SCA toxicity was assayed by trypan blue exclusion method. The highest effect/toxic- ity ratio was achieved at 100 lg mL)1 SCA; thus, we employed this concentration for most of the assays described.
Cell cultures
The cells included in the study are HaCaT cells, an immortalized human keratinocytes cell line and human dermal adult fibroblasts (NHDF Cambrex, Charles City, IA, U.S.A.). Cells were grown in F-25 flasks, or 35-mm culture dishes (Costar, Corning, NY, U.S.A.) or on 22-mm square glass coverslips placed into dishes, using Dul- becco’s modified Eagle’s medium containing 10% (v/v) foetal calf serum, 50 U mL)1 penicillin, 50 mg mL)1 streptomycin and 1% (v/v) 0.2 M l-glutamine (all from Gibco, Paisley, Scotland, U.K.). Cells were incubated at 37°C in an atmosphere containing 5% CO2.
MTT viability assay
Cell viability was documented by the MTT assay [16]. HaCaT cells cultured on 6-well Petri dishes were treated with different concentrations of SCA (0, 25, 50 and 100 lg mL)1) during several time points (2, 4, 6 and 8 days). Following these incubations, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium-bromide (MTT) solution was added to each well at a concentration of 0.5 ng mL)1, and plates were incubated at 37°C for 2–3 h. The resulting formazan crystals were dissolved by the addition of Dimethyl sulfoxide (DMSO), and absorbance was measured at 560 nm. The viability was also established by microscopy with a trypan blue exclusion test using a Neubauer chamber counter. In both cases, similar results were obtained.
Migration (wound-healing) assays
In vitro wound-healing assays were performed as previously described [15]. Briefly, HaCaT and NHDF cells were seeded in T6- well culture dishes at a density of 3 · 105 cells per well. Confluent cell monolayers were then gently scratched with a pipette tip across the entire diameter of the dish. Next, cells were rinsed with medium to remove all cellular debris, and treatment with SCA was applied to three wells, whereas the rest of the wells served as control. Cultures were observed immediately after wounding and again 12 and 24 h later, and phase-contrast pictures were taken of the wounded area using a high-resolution microscopic camera Axio- cam MRm vers.3 FireWire (D) (Zeiss, Le Pecq, France) connected to the microscope. Migrated cells were quantified by measuring the healed area. Data are given as means ± SD from three independent experiments. Statistical significance was evaluated using the Student’s t-test, and differences were considered to be significant at a value of P < 0.05 (*).
Immunostaining
For general immunodetection, cells grown on coverslips were fixed in 3% paraformaldehyde for 15 min. Samples were rehydrated in PBS, permeabilized with 1% Triton X-100 in PBS for 10 min and incubated for 1 h at 37°C with the primary mouse monoclonal antibodies against ECD, b-catenin, vinculin, FAK and phospho-FAK (BD Transduction Laboratories, Palo Alto, CA, U.S.A.) and active b-catenin (Millipore, Billerica, MA, U.S.A.) at 1 : 50 dilution in 0.1% BSA (Santa Cruz Biotechnologies, Santa Cruz, CA, U.S.A.). Afterwards, coverslips were washed with PBS, incubated with rab- bit anti-IgG of FITC-labelled secondary antibody (1 : 500 dilution in 0.1% BSA; Santa Cruz Biotechnologies) and finally washed with PBS. The preparations were mounted with Prolong (Invitrogen, Carlsbad, CA, U.S.A.). Microscopic observations and photographs were performed in an Olympus photomicroscope BX61, equipped with a HBO 100 W mercury lamp and the corresponding filter sets for fluorescence microscopy: blue (450–490, exciting filter BP 490) and green (545 nm, exciting filter BP 545). The fluorescence inten- sity of nuclear active b-catenin was measured using Imagej 1.37v software (http://rsb.info.nih.gov/ij/); at least 100 nuclei were counted for untreated (controls) and treated SCA cells, and the val- ues are given as means ± SD.
Western Blot analysis
Semiconfluent monolayers were washed and then lysed with RIPA buffer (150 mM NaCl, 1% Triton X-100, 0.05% deoxycho- late, 0.1% SDS, 1% Non idet-40, 50 mM Tris, pH 8) containing phosphatase cocktail 2 and protease inhibitor cocktail (Sigma, St. Louis, MI, U.S.A.). The samples were adjusted to the same protein concentration (BCA Protein Assay Reagent; Pierce, Rockford, IL, U.S.A.) and denatured by boiling in Laemmli sample buffer with 5% b-mercaptoethanol. 20 lg of each sample was subjected to electrophoresis separation in SDS–PAGE. Gels were then transferred to a PDVF Immobilon-P membrane (Millipore, Bedford, MA, U.S.A.) for 2 h. Membranes were stained with Ponceau S (Sigma) to control loading, and after destained, they were blocked with 5% skimmed milk in Tris-buffered saline (Tris–HCl 10 mM pH 7.6, NaCl 0.9%, 0.05% Tween 20). The membranes were incubated with the following specific antibodies: anti-ECD, vinculin, FAK, P-FAK (BD Transduction Laboratories), active b-catenin (Millipore) and G-actin (Sigma), diluted 1 : 500 in blocking buffer at 4°C overnight. HRP secondary monoclonal antibody anti-IgG of mouse was used, diluted 1 : 1000 and incubated 2 h at room temperature. Detection of bands was performed using ECL Plus Western blotting detection system (GE Healthcare, Hertfordshire, U.K.). To quantify the bands obtained by Western blot, we applied Imagej software-based analysis.
Results
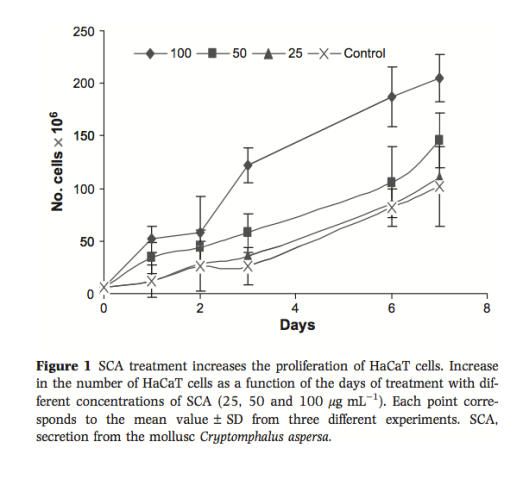
SCA treatment induces proliferation of HaCaT cells
To test whether SCA affected the proliferation of HaCaT cells, we incubated the cells with different concentrations of SCA for different times (Fig. 1). We found a non-significant increase in cell proliferation compared with control cells at 25 lg mL)1, whereas higher concentrations of SCA (50 or 100 lg mL)1) induced a gradual and significant increase with the time of treatment (Fig. 1).
SCA treatment stimulates migration of HaCaT cells and human fibroblasts
We evaluated whether exogenous treatment with SCA had an effect on the migration of keratinocytes and fibroblasts, using a classical wound-healing assay. Confluent cultures of HaCaT cells and human fibroblasts were gently scratched with a pipette tip and treated with 100 lg mL)1 SCA for 24 h. Photographs were taken before (t = 0), and 12 and 24 h after wounding (Fig. 2). As shown in Fig.2(A,C), SCA promotes rapid occupation of the space between both limits of the wound compared with control, untreated HaCaT cells. At 24 h after SCA addition, the wound was completely occupied with cells, whereas there was still an open space in control cells. Similar results were found with human dermal fibroblasts; however, closure occurred at a slower rate (Fig. 2B,C).
SCA treatment increases the expression of cell–cell and cell– substrate adhesion molecules
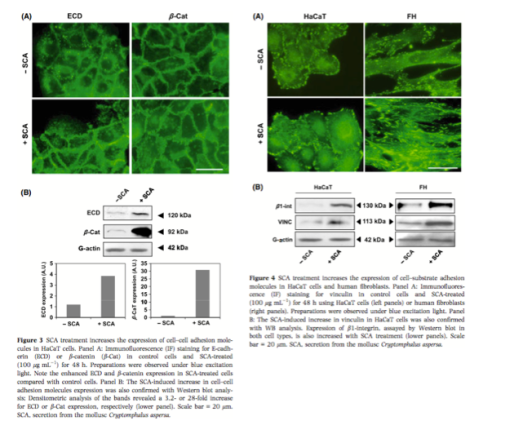
We then focused on the expression of molecules that mediates intercellular adhesion and attachment to the substrate. Immunoflu- orescence and Western blot experiments revealed that treatment of HaCaT cells with 100 lg mL)1 SCA for 48 h stimulated the expres- sion of both ECD and b-catenin (Fig. 3A). Densitrometry analysis of Western blot bands revealed a 3.2-fold increase of ECD expression and, more remarkably, a 28-fold increase for b-catenin (Fig. 3B) with SCA treatment. Moreover, SCA not only increased the expresion of ECD in keratinocytes, but it also seems to contribute to the organization of ECD in cell–cell junctions (Fig. 3A, lower panel).
To study the effect of SCA treatment in cell–matrix adhesion, we analysed the expression and distribution of the focal adhesion marker vinculin in both cultured HaCaT cells and in human fibroblasts with SCA for 48 h (100 lg mL)1). As seen in Fig. 4(A), immunofluorescence studies revealed that SCA promotes the recruitment of vinculin to focal adhesions in HaCaT cells, which correlated with increased vinculin expression as determined by Western blot analy- sis (Fig. 4B). Moreover, we also studied by Western blot the effect of SCA in b1-integrin production, a very important molecule involved in cell–substrate adhesion. Results from these experiments are shown in the lower panel of Fig. 4B, and it can be seen that SCA treatment induced the expression of vinculin and b1-integrin in HaCaT cells by 3.5- and 9.5-fold increase, respectively, compared with control, untreated cells. With respect to human fibroblast, we also found an important increase in the expression of b1-integrin and vinculin mediated by exogenous treatment with SCA, shown by both, immunofluorescence (Fig. 4A) and Western blot analysis (4- and 5.3-fold increase vs. untreated cells, Fig. 4B).
SCA treatment promotes the expression of survival molecules in keratinocytes and human fibroblasts
To assess the effect of SCA treatment on cell survival, we studied the expression of molecules involved in this process, both by immunofluorescence and Western blot. As seen in Fig. 5A (left panels), we found an increase in b-catenin expression in HaCaT cells, when treated with 100 lg mL)1 SCA for 48 h, compared with untreated cells. Furthermore, distribution of this molecule was predominantly at the nuclear area in SCA-treated cells, whereas in control cells, b-catenin was mainly located at the plasma membrane (Fig. 5A, left panels). The fluorescence intensity because of nuclear b-catenin was notably higher in SCA-treated cells compared with that of con- trols (14.06 ± 3.28 vs. 1.3 ± 0.42; Fig 5B). Western blot studies also revealed a 13.6-fold increase in protein production of active nuclear b-catenin in SCA-treated vs. control HaCaT cells (Fig. 5C).
We next investigated the expression and activation by phosphor- ylation of FAK, which is another molecule involved in cell survival, in human fibroblasts treated with SCA 100 lg mL)1 for 48 h. Immunofluorescence studies show a clear increase in both expres- sion and phosphorylation of FAK when SCA is present, compared with control cells (Fig. 5A, right panels). Comparable results were obtained by Western blot in human fibroblasts (data not shown) and in HaCaT cells (Fig. 5C). In these cells, we found that expression of FAK is increased 5-fold, whereas its phosphorylation increased 24-fold compared with control, untreated cells
Discussion
Skin ageing is a natural biological process. However, environmental aggression and genetic predisposition can accelerate this process. For example, exposure to UV wavelengths promotes oxidative dam- age, in a process referred to as photoageing. Photoageing affects the sun-exposed areas and is characterized clinically by wrinkles, roughness, dryness, laxity, telangiectasia, loss of tensile strength and pigmentary changes in the skin. There is also an increase in the development of benign and malignant neoplasms on photoaged skin. Several pathological mechanisms are involved in the process of skin ageing, such as an increase in ROS production [17], impaired remodelling capacity of the skin, reduced cell migration, adhesion and survival [1]. The search for products that can diminish the effects of either chronological ageing or UV-induced photoageing is increasing dramatically in the last few years.
Among these natural products are fern leaves [18], green tea [19], or retinoids [20]. SCA is a natural secretion from the mollusc C. aspersa endowed with regenerative properties in experimental acute radiodermatitis produced in a rat model [21]. In a recent study, Brieva et al. [15] demonstrated that SCA also exhibits antioxidant properties and promotes fibroblasts’ survival and proliferation and ECM assembly. However, the effects of SCA on keratinocyte biology or other aspects of fibroblast behaviour remained unexplored. In this report, we show that SCA induces both fibroblast and keratinocyte migration in vitro. Throughout the whole repair process, proliferation and migration of different cell types provide formation of the scar. Migration of fibroblasts, which occurs from surrounding unwounded skin towards the provisional matrix of the haemostatic plug, is a crucial step in the wound-healing process [22] that is impaired with ageing [23]. The molecular mechanisms underlying cell migration involve attachment to the ECM, rearrangement of cytoskeleton and subsequent detachment of the cell from the matrix. SCA has already been shown to induce actin reorganization bundling and microfilament alignment in human dermal fibroblasts [15].
Our results show that SCA is able to increase the migration rate of human fibroblasts as well as keratinocytes at 24 h of study, confirming its positive role in the first steps of the wound-healing process. It is of particular interest that SCA also promotes the expression of motility-related molecules, such as b1 integrins (epidermal and mucosal keratinocytes express a2b1, a3b1 and a6b4 [24], vinculin and FAK), and promotes phosphorylation of FAK, which is a mandatory step in the process of cell migration by participating in the release and reformation of adhesive contacts with the ECM [25]. Our results clearly show that SCA improves the protein production of both molecules in keratinocytes and fibroblasts, thus stimulating the attachment of these cell types to the sub- strate. Taken together, these findings suggest an important role for SCA in some of the most important events during wound healing that are weakened with age, such as cell–cell and cell–substrate adhesion. Additionally, SCA promotes nuclear appearance of b catenin. Differentiated keratinocytes are characterized by strong intercellular attachment by both desmosomes and adhesion junctions. Adherens junctions are characterized by the presence of ECD, a- and b-cate- nins, and c-catenin (plakoglobin) at the membrane [26]. b-Catenin is a multifunctional protein that plays an important role during embryonic development and neoplasia as a mediator of the Wnt signalling pathway [27]. When the Wnt pathway is quiescent, b-catenin participates in adherens junctions [28]. When b-catenin becomes cytoplasmic, it gets phosphorylated and targeted for ubiq- uitination and degradation. Activation of the Wnt pathway inhibits this phosphorylation, leading to cytosolic stabilization of b-catenin, which consequently translocates to the nucleus where it binds Tcf–Lef transcription factors and regulates transcription [29]. Once in the nucleus, b-catenin interacts with members of the Lef/Tcf family of transcription factors to activate the expression of target genes related to the cell cycle, proliferation and survival. b-catenin translocation to nucleus is associated with several epithelial can- cers [30], because it inhibits apoptosis signals and promotes migration and proliferation of epithelial cells [10]. Moreover, in a recent report, it was demonstrated that UVB radiation causes apoptosis, and this process was directly related with cleavage of ECD and its downstream components, including b-catenin [31]. Our results indicate a clear increase in the expression of nuclear b-catenin in cultured keratinocytes after treatment with SCA. This observation clearly points to the ability of SCA in inhibiting keratinocyte apop- tosis, although additional studies should be performed.
In addition to its role in regulating cell motility, phosphorylation of FAK also mediates cell survival [32]. At focal adhesions, inte- grins can activate various protein tyrosine kinases, including FAK. The cytoplasmic tail of integrin b subunits activate FAK, and then, it autophosphorylates Tyr397 creating a binding site for several pro- teins. These proteins eventually promote signalling pathways that modify the cytoskeleton and activate the extracellular signal-regulated kinase (ERK) family of mitogen-activated protein kinases, leading to cell proliferation [32]. According to Manohar et al., [33] b1 integrin promotes keratinocyte survival through activation of the ERK cascade, also involving phosphorylation of FAK. Here, we have shown that SCA induces an increase in the expression of FAK and P-FAK in cultured keratinocytes. Thus, through increased expression of b1 integrin and phosphorylated FAK, SCA plays a dual role in promoting cytoskeletal reorganization and promoting cell survival.
In summary, our results provide new insights into molecular mechanisms underlying the regenerative properties of SCA. Furthermore, they may suggest dermatologic or cosmetic uses of SCA-based products as to diminish ageing-induced cutaneous manifestations.
Acknowledgements
The authors declare that the main subject of this research (SCA) is subject to US patent US 5538740. The study was partially sup- ported by a research grant from Industrial Farmace ́utica Cantabria, S.A., Madrid, Spain. S.G. is a consultant for I.F.C.
References
1. Ashcroft, G.S., Horan, M.A. and Ferguson, M.W. The effects of ageing on cutaneous wound healing in mammals. J. Anat.
2. auf dem Keller, U., Kumin, A., Braun, S. and Werner, S. Reactive oxygen species and their detoxification in healing skin wounds. J. Investig. Dermatol. Symp. Proc. 11, 106– 111 (2006)
3. Watson, R.E. and Griffiths, C.E. Pathogenic aspects of cutaneous photoaging. J. Cosmet. Dermatol. 4, 230–236 (2005).
4. Neville, J.A., Welch, E. and Leffell, D.J. Man- agement of nonmelanoma skin cancer in 2007. Nat. Clin. Pract. Oncol. 4, 462–469 (2007)
5. Ericson, M.B., Wennberg, A.M. and Larko, O. Review of photodynamic therapy in acti- nic keratosis and basal cell carcinoma. Ther. Clin. Risk Manag. 4, 1–9 (2008).
6. Knudsen, K.A. and Soler, A.P. Cadherin- mediated cell-cell interactions. Methods Mol. Biol. 137, 409–440 (2000).
7. Yamada, K.M. and Miyamoto, S. Integrin transmembrane signaling and cytoskeletal control. Curr. Opin. Cell Biol. 7, 681–689 (1995).
8. Zouq, N.K., Keeble, J.A., Lindsay, J. et al. 16. FAK engages multiple pathways to maintain survival of fibroblasts and epithelia – differ- ential roles for paxillin and p130Cas. J. Cell Sci. 122(Pt 3), 357–367 (2009).
9. Olmeda, D., Castel, S., Vilaro, S. and Cano, A. {beta}-Catenin regulation during the cell cycle: implications in G2/M and apoptosis. Mol. Biol. Cell 14, 2844–2860 (2003).
10. Fuchs, S.Y., Ougolkov, A.V., Spiegelman, V.S. and Minamoto, T. Oncogenic beta-cate- nin signaling networks in colorectal cancer. Cell Cycle 4, 1522–1539 (2005).
11. Badiu, D.L., Balu, A.M., Barbes, L., Luque, R., Nita, R., Radu, M., Tanase, E. and Rosoiu, N. Physico-chemical characterisation of lipids from Mytilus galloprovincialis (L.) and Ra- pana venosa and their healing properties on skin burns. Lipids 43, 829–841 (2008).
12. Tsoutsos, D., Kakagia, D. and Tamparopou- los, K. The efficacy of Helix aspersa Muller extract in the healing of partial thickness burns: a novel treatment for open burn management protocols. J. Dermatolog. Treat. 20, 1–5 (2009).
13. Brieva, A.G., Guerrero, A. and Pivel, J.P. Antioxidative properties of a mollusc secre- tion (SCA): a skin protective product. Methods Find. Exp. Clin. Pharmacol (Suppl. A), 175 (1999).
14. Ledo, E., de las Heras, M.E. and Ledo, A. Treatment for acute radiodermatitis with Cryptomphalus aspersa secretion. Radio- proteccio ́n 23, 34–38 (1999)
15. Brieva, A., Philips, N., Tejedor, R., Guerrero, A., Pivel, J.P., Alonso-Lebrero, J.L. et al. Molecular basis for the regenerative properties of a secretion of the mollusk Cryptomph-alus aspersa. Skin Pharmacol. Physiol. 21, 15–22 (2008).
16. Merlin, J.L., Azzi, S., Lignon, D., Ramacci, C., Zeghari, N. and Guillemin, F. MTT assays allow quick and reliable measure- ment of the response of human tumour cells to photodynamic therapy. Eur. J. Cancer 28A, 1452–1458 (1992)
17. Harman, D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 11, 298–300 (1956).
18. Capote, R., Alonso-Lebrero, J.L., Garcia, F., Brieva, A., Pivel, J.P. and Gonzalez, S. Polypodium leucotomos extract inhibits trans- urocanic acid photoisomerization and photo- decomposition. J. Photochem. Photobiol. B 82, 173–179 (2006).
19. Farris, P. Idebenone, green tea, and Coffee- berry extract: new and innovative antioxi- dants. Dermatol. Ther. 20, 322–329 (2007)
20. Mukherjee, S., Date, A., Patravale, V., Kor- ting, H.C., Roeder, A. and Weindl, G. Reti- noids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin. Interv. Aging 1, 327–348 (2006).
21. Abad, R. Treatment of experimental radio- dermatitis with a regenerative glucoproteic mucopolysaccharide complex. Dermatol. Cosmet. 9, 53–57 (1999).
22. Clark, R.A.F. Cutaneous tissue repair: basic biologic considerations. I. J. Am. Acad. Dermatol. 13(5, Pt 1), 701–725 (1985).
23. Reed, M.J., Ferara, N.S. and Vernon, R.B. Impaired migration, integrin function, and actin cytoskeletal organization in dermal fibroblasts from a subset of aged human donors. Mech. Ageing Dev. 122, 1203–1220 (2001).
24. De Luca, M., Tamura, R.N., Kajiji, S., Bondanza, S., Rossino, P., Cancedda, R.et al. Polarized integrin mediates human keratinocyte adhesion to basal lamina. Proc. Natl. Acad. Sci. U S A 87, 6888– 6892 (1990).
25. Webb, D.J., Donais, K., Whitmore, L.A., Thomas, S.M., Turner, C.E., Parsons, J.T. et al. FAK-Src signalling through paxillin, ERK and MLCK regulates adhesion disas- sembly. Nat. Cell Biol. 6, 154–161 (2004).
26. Kemler, R. From cadherins to catenins: cyto- plasmic protein interactions and regulation of cell adhesion. Trends Genet. 9, 317–321 (1993).
27. Millar, S.E. WNTs: multiple genes, multiple functions. J. Invest. Dermatol. 120, 7–8 (2003).
28. Geiger, B. and Ayalon, O. Cadherins. Annu. Rev. Cell Biol. 8, 307–332 (1992)
29. Hecht, A. and Kemler, R. Curbing the nuclear activities of beta-catenin. Control over Wnt target gene expression. EMBO Rep. 1, 24–28 (2000).
30. Palacios, J. and Gamallo, C. Mutations in the {beta}-Catenin Gene (CTNNB1) in endo- metrioid ovarian carcinomas. Cancer Res. 58, 1344–1347 (1998).
31. Hung, C.-F., Chiang, H.S., Lo, H.M., Jian, J.S. and Wu, W.B. E-cadherin and its down- stream catenins are proteolytically cleaved in human HaCaT keratinocytes exposed to UVB. Exp. Dermatol. 15, 315–321 (2006).
32. Giancotti, F.G. and Ruoslahti, E. Integrin signaling. Science 285, 1028–1033 (1999).
33. Manohar, A., Shome, S.G., Lamar, J., Stir- ling, L., Iyer, V., Pumiglia, K. etal. {alpha}3{beta}1 integrin promotes kerati- nocyte cell survival through activation of a MEK/ERK signaling pathway. J. Cell Sci. 117, 4043–4054 (2004).